
מונוכרום Shift+A
ניגודיות כהה Shift+S
ניגודיות בהירהShift+D
הגדל גופן Shift+F
הקטן גופן Shift+Z
הדגשת קישורים Shift+X
איפוסShift+C
הצהרת נגישות
© כל הזכויות שמורות 2018







הצהרת נגישות
© כל הזכויות שמורות 2018
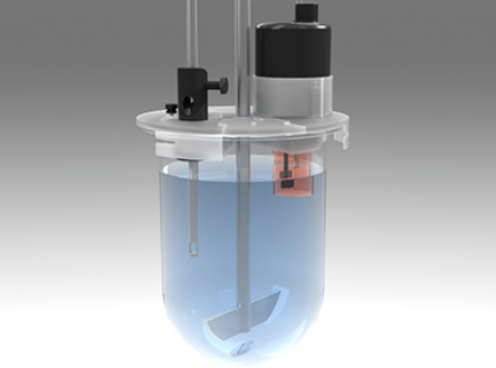
Achieve more realistic IVIVC modeling with better in situ concentration monitoring.
In many cases, dissolution experiments alone cannot correctly predict the in vivo response to drug products due to the complicated interplay of solubility and permeability in complex media. Pion’s MacroFLUX device extends the utility of in situ concentration monitoring to improve assessment of absorption potential and more realistic IVIVC modeling.
Introducing a stirred absorption chamber into the traditional USP I and II apparatus allows this type of testing to be done in vitro through the use of the MacroFLUX dissolution system. The USP vessel is the donor compartment, allowing for the volumes needed to test finished dosage forms under sink conditions. Using in situ fiber optic UV detection in both the donor and receiver provides the required data density for accurate assessment of transmembrane FLUX.
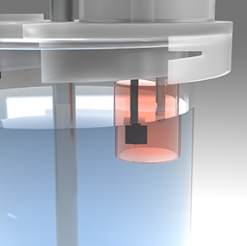
The patented MacroFlux technology provides real time IVIVC prediction as the next step in Pion FLUX family of products.
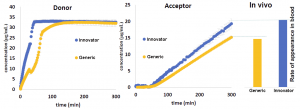
Dissolution profile (on the left), in vitro appearance profile (in the middle), and in vivo appearance (on the right) of active ingredient from different formulations.
For more information about MacroFLUX